PRODUCTION OF RECOMBINANT, NON-TAGGED PHENYLALANINE AMMONIA-LYASES EMPLOYING TEV PROTEASE-REMOVABLE AFFINITY TAGS
DOI:
https://doi.org/10.24193/subbchem.2022.4.03Keywords:
affinity tags, phenylalanine ammonia-lyase, TEV (Tobacco Etch Virus) protease, thermostability, specific enzyme activity, oligomerization state.Abstract
Nowadays, protein purification by the aid of affinity tags can be carried out with high speed and efficiency. However, in several cases, affinity tags can significantly alter the key properties of enzymes, especially activity and/or thermostability.
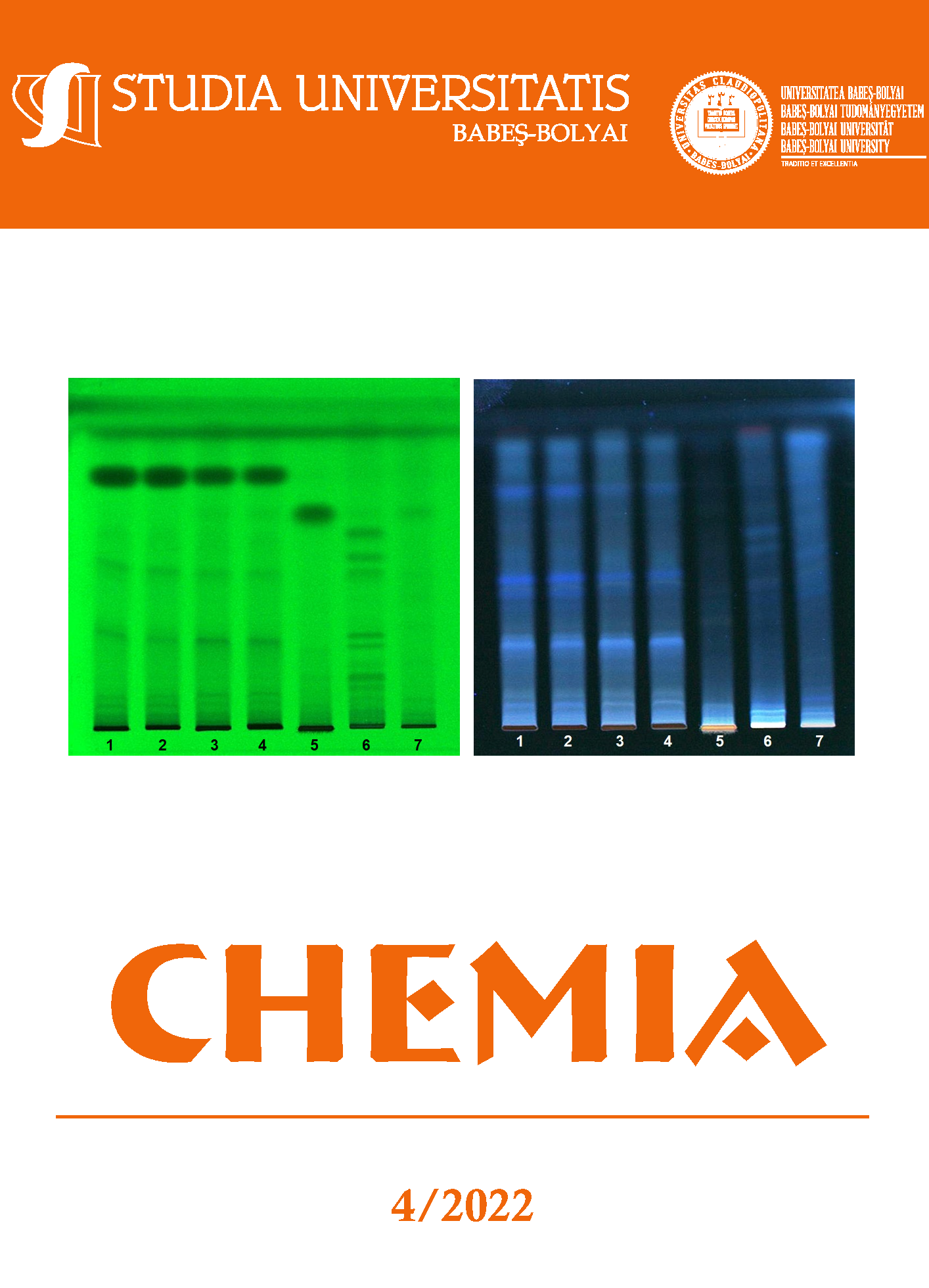
This study focused on the purification of the non-tagged phenylalanine ammonia-lyase from Petroselinum crispum (PcPAL), as well as on the purification of the TEV (Tobacco Etch Virus) protease, the molecular scissors used to remove the affinity tag from the recombinantly expressed PcPAL. Removal of the 6xHis-tag led to a 1.5-fold increase in the specific activity of PcPAL, while the absence of the affinity tag did not significantly alter the thermostability of the protein. The purity and oligomerization state of the proteins of interest were also analyzed by size exclusion chromatography, both before and after the removal of the affinity tag, confirming the stability of the tetrameric fold of PcPAL.
References
D. Walls; S.T. Loughran, Protein Chromatography: Methods and Protocols, Methods Mol. Biol., 2011, 681, 151–175.
S. Van den Berg; P.A. Lofdahl; T. Hard; H. Berglund, J. Biotechnol. 2006, 121, 291–298.
S. Harper; D.W. Speicher, Methods Mol. Biol., 2011, 681, 259–280.
A. Einhauer; A. Jungbauer, J. Biochem. Biophys. Meth., 2001, 49, 1–3, 455–465.
J.J. Lichty; J.L. Malecki; H.D. Agnew; D.J. Michelson-Horowitz; S. Tan, Protein Expr. Purif., 2005, 41, 1, 98–105.
J. Arnau; C. Lauritzen; G.E. Petersen; J. Pedersen, Protein Expr. Purif., 2006, 48 (1), 1–13.
H. Nam; B-J. Hwang; D-Y. Choi; S. Shin; M. Choi, FEBS Open Bio., 2020, 10, 619–626.
W.T. Booth; C.R. Schlachter; S. Pote; N. Ussin; N.J. Mank; V. Klapper et al., ACS Omega, 2018, 3, 760−768.
D.M. Charbonneau; F. Meddeb-Mouelhi; M. Beauregard, Protein Pept. Lett., 2012, 19, 264-269.
V.P. Kutyshenko; G.V. Mikoulinskaia; S.V. Chernyshov; A.Y. Yegorov; D.A. Prokhorov; V.N. Uversky, Int. J. Biol. Macromol., 2019, 124, 810–818.
E. Niedzialkowska; O. Gasiorowska; B.K. Handing; A.K. Majorek; J.P. Porebski; G.I. Shabalin; E. Zasadzinska; M. Cymborowski; W. Minor; Protein Sci., 2016, 25, 720−733.
C. Han; Q. Wang; Y. Sun; R. Yang; M. Liu; S. Wang et al, Biotechnol. Biofuels, 2020, 13:30, 2223.
M. Carson; H.D. Johnson; H. McDonald; C. Brouillette; J.L. Delucas, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2007, 63, 295−301.
M.C. Deller; L. Kong; B. Rupp, Acta Crystallogr., Sect. F: Struct. Biol. Commun., 2016, 72, 72−95.
M.E. Kimple; J. Sondek, Curr. Protoc. Prot. Sci., 2018, 73, 1, 9.9.1–9.9.19.
G. Lilius; M. Persson; L. Bulow; K. Mosbach, Eur. J. Biochem. 1991, 198, 499–504.
M.C. Smith; T.C. Furman; T.D. Ingolia; C. Pidgeon, J. Biol. Chem., 1988, 263, 7211–7215.
H. Block; B. Maertens; A. Spriestersbach; N. Brinker; J. Kubicek; R. Fabis, et al., Methods Enzymol. 2009, 463, 439–473.
a) N.A. Dima; A. Filip; L.C. Bencze; M. Oláh; P. Sátorhelyi; B.G. Vértessy et al., Stud. Univ. Babes-Bolyai Chem., 2016, 61, 21–34. b) A. Filip; L.C. Bencze; C. Paizs; L. Poppe; F.D. Irimie, Stud. Univ. Babes-Bolyai Biol. 2015, 60(Sp. Iss), 39–43. c) P. Csuka; V. Juhász; S. Kohári; A. Filip; A. Varga; P. Sátorhelyi; et al., ChemBioChem, 2018, 19, 1–9. d) A. Varga; Z. Bata; P. Csuka; M.D. Bordea; B.G. Vértessy; A. Marcovici; F.D. Irimie, L. Poppe, L.C. Bencze; Stud. Univ. Babes-Bolyai Chem., 2017, 3, 293–308. e) A. Varga; P. Csuka; O. Sonesouphap; G. Bánóczi, M.I. Toşa; G. Katona, et al., Catal. Today, 2021, 366, 185–194. f) K. Kovács; G. Bánóczi; A. Varga, I. Szabó; A. Holczinger; G. Hornyánszky; I. Zagyva; C. Paizs; B.G. Vértessy, L. Poppe; PLoS ONE 2014, 9, e85943.
N. Debeljak; L. Feldman; K.L. Davis; R. Komel, A.J. Sytkowski, Anal. Biochem. 2006; 359(2), 216–223.
H.C. Wilken; S. Rogge; O. Götze; T. Werfel; J. Zwirnera, J. Immunol. Meth., 1999, 226(1-2), 139–145.
Y-T. Lai; Y-Y. Chang; L. Hu; Y. Yang; A. Chao; Z-Y. Du, et al., PNAS, 2015, 112(10), 2948–2953.
K.A. Majorek; M.L. Kuhn; M. Chruszcz; F.W. Anderson; W. Minor; Protein Sci., 2014, 23, 1359−1368.
A. Panek; O. Pietrow; P. Filipkowski; J. Synowiecki; J., Acta Biochim. Pol., 2013, 60, 163−166.
A.P.U. Araújo; G. Oliva; F. Henrique-Silva; C.R. Garratt; O. Caceres; M.L. Beltramini, Biochem. Biophys. Res. Commun. 2000, 272, 480−484.
W. Xiao; L. Jiang; W. Wang; R. Wang; J. Fan, J. Biosci. Bioeng., 2018, 125(2), 160−167.
E.Z.A. Nagy, S.D. Tork; L.A. Pauline; A. Filip; F.D. Irimie; L. Poppe et al. ACS Catal. 2019, 9, 8825–8834.
S.H.J. Smits; A. Mueller; M.K. Grieshaberb; L. Schmitt, Acta Cryst. F: Struct. Biol. Commun., 2008, 64, 836–839.
a) A. Filip, Wild-type and tailored phenylalanine ammonia-lyases for the synthesis of unnatural L- and D-arylalanines (Doctoral Thesis), Babeş-Bolyai University of Cluj-Napoca, 2019, 50-68. b) Z. Bata; Z. Molnár; E. Madaras; B. Molnár; E. Sánta-Bell; A. Varga et al., ACS Catal., 2021, 11, 4538−4549.
A.J. Barrett; J.K. McDonald, Biochem. J., 1986, 237, 935.
J.A. Mótyán; F. Tóth; J. Tőzsér; Biomolecules, 2013, 3, 923–942.
C.S. Craik; J.M. Page; L.E. Madison, Biochem. J., 2011, 435, 1−16.
B. Miladi; H. Bouallagui; C. Dridi; A. El Marjou; G. Boeuf; P. Di Martino, et al. Protein Expr. Purif., 2011; 75(1), 75−82.
P. Giansanti; L. Tsiatsianiv Y.T. Low; J.A. Heck, Nat. Protoc. 2016, 11, 993−1006.
F. Xiao; Widlak, W.T. Garrard; Nucleic Acids Res. 2007, 35(13), 1−7.
K. Melcher, Anal. Biochem., 2000, 277, 109–120.
a) J.L. Riechmann; S. Lain, J.A. Garcia; J. Gen. Virol., 1992, 73, 1–16. b) W.G. Dougherty; B.L. Semler; Microbiol. Rev. 1993, 57, 781–822. c) R.B. Kapust; J. Tozser; J.D. Fox; D.E. Anderson; S. Cherry; T.D. Copeland; D.S. Waugh; Protein Eng., 2001, 12, 993–1000. d) L.D. Cabrita; G. Dimitri; A.L. Robertson; Y. Dehouck; M. Rooman; S.P. Bottomley, Protein Sci., 2007, 16, 2360–2367.
A. Zlobin and A. Golovin, ACS Omega 2022, 7, 44, 40279–40292.
a) D.S. Waugh; Protein Expr. Purif. 2011, 80, 283–293; b) A.C. Denard; C. Paresi; R. Yaghi; N. McGinnis; Z. Bennett; L. Yi, G. Georgiou; B.L. Iverson, ACS Synth. Biol. 2021, 10, 63−71; c) H. Mohammadian; K. Mahnam; H. Mirmohammad Sadeghi; M.R. Ganjalikhany V. Akbari, RPS 2020, 15(2), 164–173.
T.S. Ahmed; F. Parmeggiani; N.J. Weise; S.L. Flitsch; N.J. Turner, ACS Catal., 2018, 8, 3129–3132.
a) A. Filip, E.Z.A. Nagy; S.D. Tork; G. Bánóczi. M.I. Toșa; F.D. Irimie et al. ChemCatChem., 2018, 10, 2627–2633; b) S.D. Tork; E.Z.A. Nagy; L. Cserepes; D.M. Bordea; B. Nagy; M.I. Toșa; et al. Sci. Rep., 2019, 9, 1; c) S.D. Tork; M.E. Moisa; L. Cserepes; A. Filip; L.C. Nagy; F.D. Irimie; L.C. Bencze, Sci. Rep., 2022, 12(1), 10606; d) L.C. Bencze, A. Filip; G. Bánóczi; M.I. Toșa F.D. Irimie; A. Gellért et al., Org. Biomol. Chem., 2017, 15, 3717–3727.
LA. Hardegger; P. Beney; D. Bixel; C. Fleury; F. Gao; AGG. Perrenoud et al., Org. Proc. Res. Dev., 2020, 24(9), 1763–1771.
F. Parmeggiani; N.J. Weise; S.T. Ahmed; N.J. Turner, Chem. Rev. 2018, 118, 73–118.
E.Z.A. Nagy; S.D. Tork; A Filip; L. Poppe; M.I. Tosa; C. Paizs; L.C. Bencze; Other Carbon–Nitrogen Bond-Forming Biotransformations, Production of L- and D- phenylalanine analogues using tailored phenylalanine ammonia-lyases in Applied Biocatalysis, The Chemist's Enzyme Toolbox, J. Whittall and Peter W. Sutton; Wiley, Hoboken, USA, 2021, Chapter 5; 5.5; 42 (4), 215-221. ISBN: 978-1-119-48701-2.
a) D. Duță; A. Filip; L.C. Nagy; E.Z.A. Nagy; R. Tőtős; L.C. Bencze, Sci. Rep., 2022, 12, 3347. b) E.Z.A. Nagy; L.C. Nagy; A. Filip; K. Nagy; E. Gál; R. Tőtős; L. Poppe; C. Paizs; L.C. Bencze; Sci. Rep., 2019, 9, 1–10.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Studia Universitatis Babeș-Bolyai Chemia

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.