SIMULTANEOUS DETERMINATION OF CALCIUM AND MAGNESIUM IN NATURAL WATERS BY METHANE-AIR FLAME EMISSION AND FLAME ATOMIC ABSORPTION SPECTROMETRY USING A MICROSPECTROMETER
Keywords:
methane-air flame, FAES, FAAS, CCD microspectrometer, Ca, Mg, simultaneous determinationAbstract
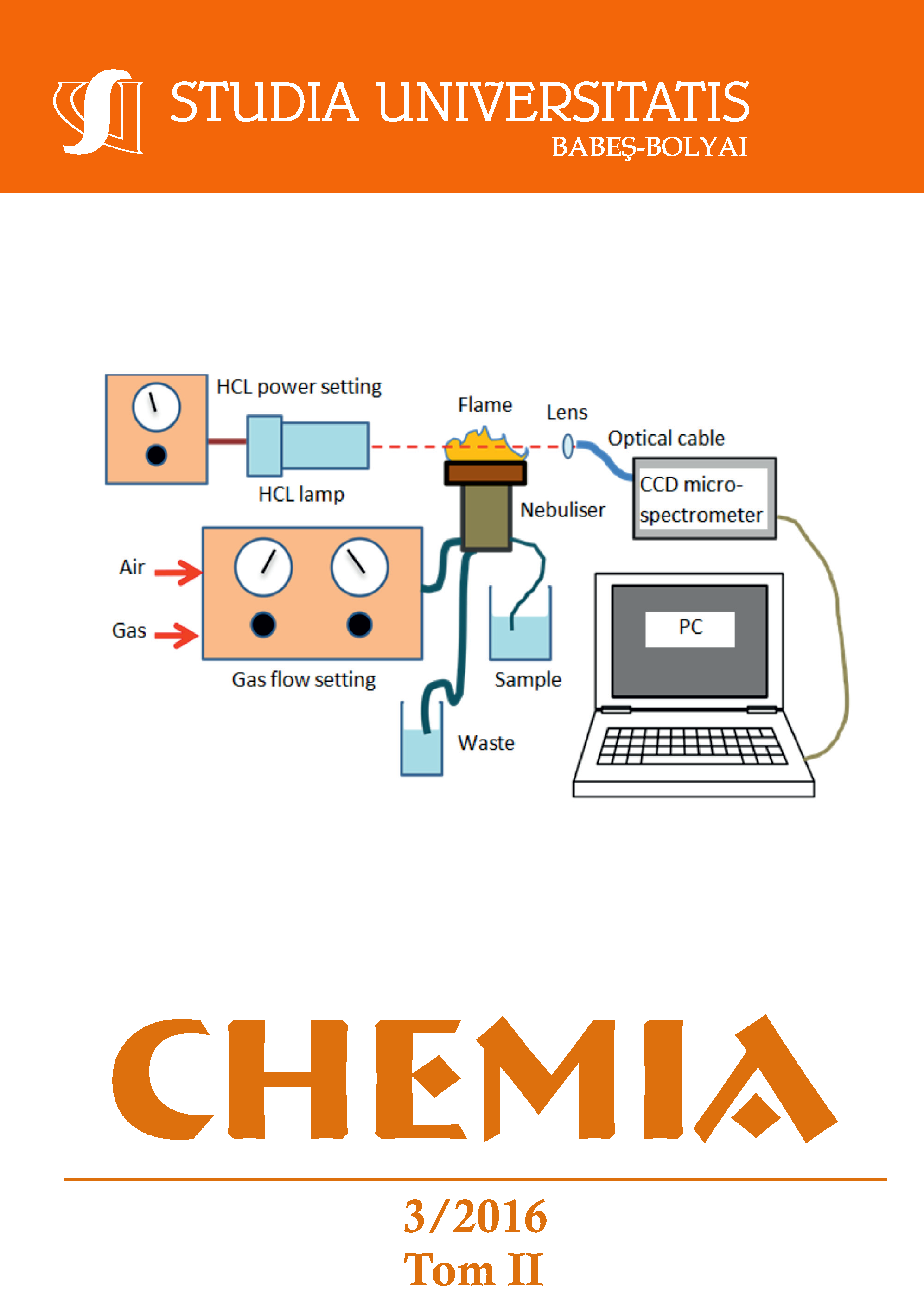
The calcium and magnesium content of liquid samples has been determined directly by flame atomic absorption (FAAS) and flame atomic emission (FAES) spectrometry using the methane-air (M-A) flame. We measured simultaneously the intensity of the 554 nm wavelength molecular bands emitted by excited CaOH molecules and the decrease of light intensity of a hollow cathode lamp (HCL) absorbed by ground state Mg atoms. The simultaneous multiwavelength measurements enhanced by a charge-coupled device (CCD) microspectrometer allowed fast background correction for each studied element. The instrumental and flame parameters were optimized; the best results were obtained using a lamp current of 1 mA, and an observation height of 5 mm, in case of a reducing flame. The calcium and magnesium content of bottled water samples and water standard certified reference material (CRM) have been determined with standard addition method. The recovery for CRM was 97.80% for Ca and 98.51% for Mg. Under optimal working conditions the detection limits (according to the 3s criterion) were 25 µg·L-1 for Ca and 5.4 µg·L-1 for Mg.
References
A. Zsigmond, “Minőségi és mennyiségi analitikai kémia laborkönyv”, Editura Scientia, 2008, chapter 1-3.
L. Noel, M. Carl, C. Vastel, T. Guerin, International Dairy Journal, 2008, 18, 899.
Leo M.L. Nollet, Leen S. P. De Gelder, “Handbook of Water Analysis”, Third edition, CRC Press, 2014, chapter 15.
L. Kékedy Nagy, E. A. Cordoş, Studia UBB Chemia, 1999, 44, 137.
L.Kékedy-Nagy, E. A. Cordoş, Studia UBB Chemia, 1999, 44, 183.
C. Prohaska, K. Pomazal, I. Steffan, Fresenius' Journal of Analytical Chemistry, 2000, 367, 479.
D. Beauchemin, J. W. McLaren, A. P. Mykytiuk, S. S. Berman, Anal. Chem., 1987, 59, 778.
Z. Kilic, O. Acar, M. Ulasan and M. Tlim, Food Chem., 2002, 76, 107.
C. Bendicho, I. Calle, F. Pena, N. Cabaleiro, I. Lavilla, Trends Anal. Chem., 2012, 31, 50.
C. Bendicho, I. Lavilla, F. Pena-Pereira, V. Romero, J. Anal. At. Spectrom., 2012, 27, 1831.
C. Zheng, Y. He, S. Weia, X. Hou, J. Anal. At. Spectrom., 2005, 20, 60.
A. R. Zsigmond, T. Frentiu, M. Ponta, M. Frentiu, D. Petreus, Food Chemistry, 2013, 141, 3621.
T. Frentiu, E. Darvasi, S. Butaciu, M. Ponta, D. Petreus, R. Etz, M. Frentiu, Microchem. J. 2015, 121, 192.
T. Frentiu, E. Darvasi, S. Butaciu, M. Ponta, D. Petreus, A. I. Mihaltan, M. Frentiu, Talanta, 2014, 129, 72.
G. Tyler “AA or ICP – Which do you choose?” Varian Australia Pty. Ltd., ICP–3, Melbourne, Australia, 1991.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2016 Studia Universitatis Babeș-Bolyai Chemia

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.