ALUMINUM PILLARED BENTONITE – CHARACTERIZATION AND SYNTHESIS OPTIMIZATION BY RESPONSE SURFACE METHODOLOGY
DOI:
https://doi.org/10.24193/subbchem.2021.1.06Keywords:
bentonite, mathematical modelling, pillared clay, Response Surface Methodology.Abstract
Bentonite is a clay mineral whose chemical structure can be easily modified by pillaring process for introduction of various cations such as aluminum, chromium, nickel, zinc etc. fact that conducts to attractive and versatile products suitable for diverse applications going from gas separation to pollutants removal or excipients for food industry for example.
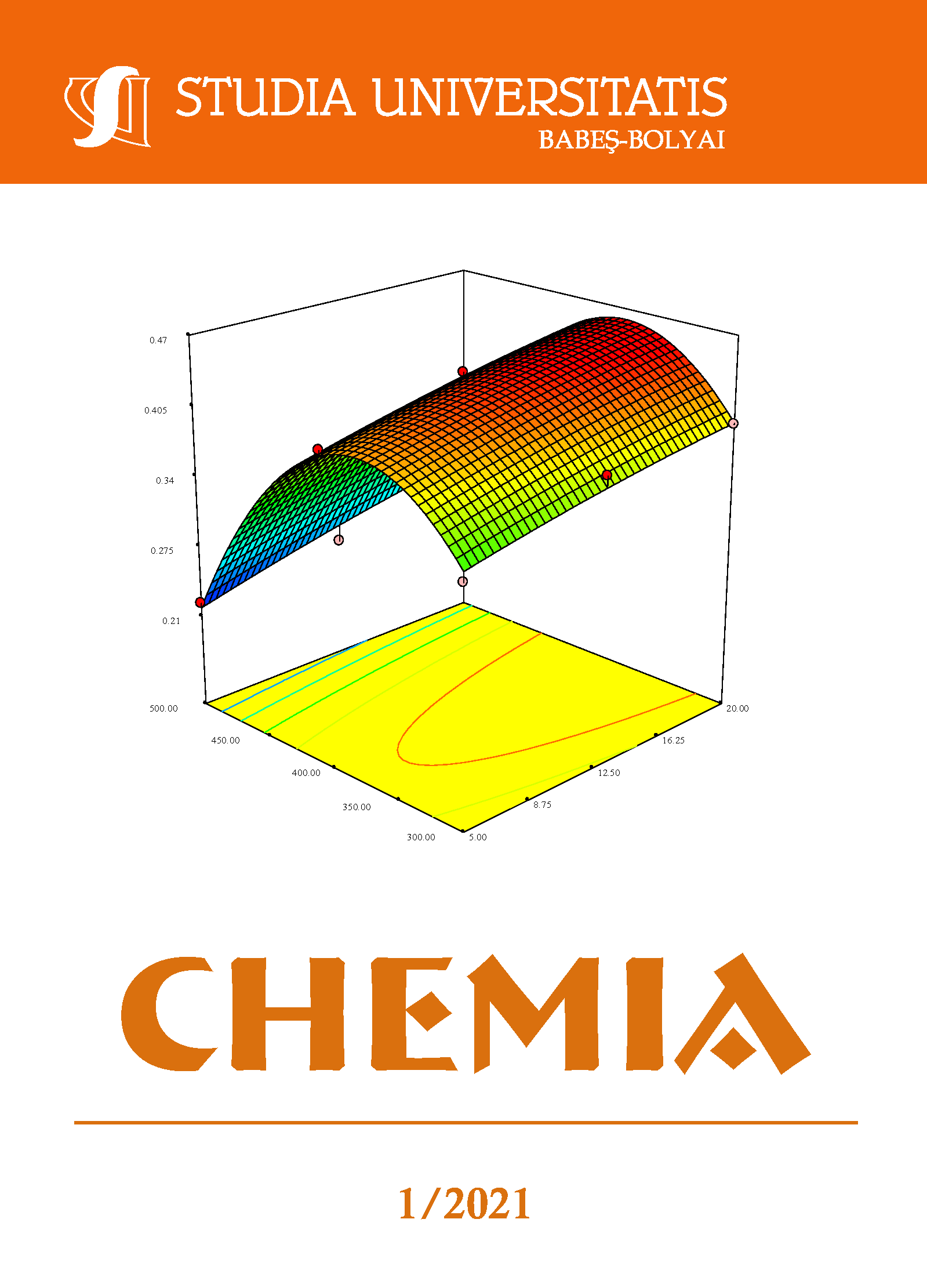
This paper deals with the synthesis of aluminum pillared bentonite based on a process involving bentonite suspension and pillaring agent preparation, bentonite intercalation and calcination. The raw material and the obtained products were analyzed by X-ray diffraction, nitrogen adsorption-desorption technique, ammonia-temperature programmed desorption and scanning electronic microscopy. Three ratios of aluminum cations – amount of bentonite (5 mmol/g, 12.5 mmol/g, 20 mmol/g) and three calcination temperatures (300 °C, 400 °C, 500 °C) were used according to a Response Surface Methodology program aiming to attain the highest interlamellar distance, specific surface area and surface acidity. Collected data were fitted to second order polynomial equations. An aluminum cation – bentonite amount ratio of 12.5 mmol/g and a calcination temperature of 400 °C were found as appropriate conditions for bentonite pillaring process. Tests conducted on these settings showed that mathematical models were in good agreement with the experimental values presenting a high degree of accuracy.
References
A.V. Ursu; G. Jinescu; F. Gros; I.D. Nistor; N.D. Miron; G. Lisa; M. Silion; G. Djelveh; A. Azzouz; J. Therm. Anal. Calorim., 2011, 106(3), 965-971.
F. Rouquerol; J. Rouquerol; K. Sing; Adsorption by powders and porous solids. Principles, methodology and applications, Academic Press, London, 1999, pp. 357-378.
R.A. Schoonheydt; Appl. Clay Sci., 2016, 131, 107-112.
C.H. Zhou; L.Z. Zhao; A.Q. Wang; T.H. Chen; H.P. He; Appl. Clay Sci., 2016, 119, 3-7.
P.S.C. Silva; S.M.B. Oliveira; L. Farias; D.I.T. Fávaro; B.P. Mazzilli; Appl. Clay Sci., 2011, 52(1), 145-149.
T.J. Gutiérrez; A.G. Ponce; V.A. Alvarez; Mater. Chem. Phys., 2017, 194, 283-292.
J. Konta; Appl. Clay Sci., 1995, 10(4), 275-335.
F.J. Galindo-Rosales; F.J. Rubio-Hernández; Appl. Clay Sci., 2006, 33(2), 109-115.
L. Gu; J. Xu; L. Lv; B. Liu; H. Zhang; X. Yu; Z. Luo; Desalination, 2011, 269(1), 206-213.
M. El Bouraie; A.A. Masoud; App. Clay Sci., 2017, 140, 157-164.
Z. Vryzas; V.C. Kelessidis; L. Nalbantian; V. Zaspalis; D.I. Gerogiorgis; Y. Wubulikasimu; Appl. Clay Sci., 2017, 136, 26-36.
M.K. Uddin; Chem. Eng. J., 2017, 308, 438-462.
J.N. Putro; S.P. Santoso; S. Ismadji; Y.-H. Ju; Microporous Mesoporous Mater., 2017, 246, 166-177.
I. Belbachir; B. Makhoukhi; J. Taiwan Institute Chem. Eng., 75, 2017, 105-111.
N. Belhouchat; H. Zaghouane-Boudiaf; C. Viseras; Appl. Clay Sci., 2017, 135, 9-15.
X. Liang; Y. Lu; Z. Li; C. Yang; C. Niu; X. Su; Microporous Mesoporous Mater., 2017, 241, 107-114.
K. Styszko; K. Nosek; M. Motak; K. Bester; C. R. Chim., 2015, 18(10), 1134-1142.
J. Fan; W. Yang; A. Li; React. Funct. Polym., 2011, 71(10), 994-1000.
T. Ngulube; J.R. Gumbo; V. Masindi; A. Maity; J. Environ. Manag., 2017, 191, 35-57.
T. Xu; Y. Liu; F. Ge; L. Liu; Ouyang, Y., Appl. Surf. Sci., 2013, 280, 926-932.
A.V. Ursu; C. Jinescu; I.D. Nistor; V.A. Aruș; G. Isopencu; M.A. Mareș; Rev. Chim., 2010, (61)12, 1226-1230.
S. Nousir; V.A. Aruș; T.C. Shiao; N. Bouazizi; R. Roy; A. Azzouz; Microporous and Mesoporous Mater., 2019, 290, DOI: 10.1016/j.micromeso.2019.109652.
F. Boudissa; D. Mirilă; V.A. Aruș; T. Terkmani; S. Semaan; M. Proulx; I.D. Nistor; R. Roy; A. Azzouz; J. Hazard. Mater.; 2019, 364(15), 356-366.
R.A. Schoonheydt; T. Pinnavaia; G. Lagaly; N. Gangas; Pure Appl. Chem., 1999, 71(12), 2367-2371.
R.A. Schoonheyat; T. Pinnavia; G. Lagaly; N. Gangas; Pure Appl. Chem., 1999, 71, 2367-2371.
S. Nousir; V.A. Aruș; T.C. Shiao; N. Bouazizi; R. Roy; A. Azzouz; Microporous Mesoporous Mater., 2019, 290, 109652.
G. Muntianu; N. Platon; A. Mardaru; I.D. Nistor; N.D. Miron; G. Jinescu; U.P.B. Sci. Bull., Series B, 2015, 77(3), 151-164.
D.C. Mirilă; M.Ș. Pîrvan; N. Platon; A.M. Georgescu; V. Zichil; I.D. Nistor; Acta Chem. Iași, 2018, 26(2), 263-280.
R. Azzallou; R. Mamouni; Y. Riadi; M. El Haddad; Y. El Mouzdahir; R. Mahboub; A. Elmchaouri; S. Lazar; G. Guillaumet; Rev. Chim., 2010, 61(12), 1155-1157.
A.-M. Georgescu; G. Brabie; I.D. Nistor; C. Penot; F. Nardou; J. Porous Mater., 2015, 22(4), 1009-1019.
D.C. Mirilă; M.S. Pârvan; A.M. Roșu; V. Zichil; I.D. Nistor: Sci. St. Res. Chem. Chem. Eng. Biotechnol. Food Ind., 2018, 19(1), 63-72.
M.L. Chavez-Garcia; L. De Pablo-Galan; M.P. Saucedo-Ramirez; J. Mex. Chem. Soc., 2006, 50(1), 36-41.
P. Cañizares; J.L. Valverde; M.R. Sun Kou; C.B. Molina; Microporous Mesoporous Mater., 1999, 29(3), 267-281.
J.T. Kloprogge; L.V. Duong; R.L. Frost; Environ. Geol., 2005, 47(7), 967-981.
S. Sivakumar; S.K. Ghosh; A.D. Damodaran; K.G.K. Warrier; Mater. Lett., 1997, 31(1), 113-118.
S. Vercauteren; J. Luyten; R. Leysen; E.F. Vansant; J. Membr. Sci., 1996, 119(1), 161-168.
A.A.G. Tomlinson; J. Porous Mater., 1998, 5(3), 259-274.
A. Gil; L.M. Gandı́a; Chem. Eng. Sci., 2003, 58(14), 3059-3075.
H.J. Chae; I.-S. Nam; S.W. Ham; S.B. Hong; Catal. Today, 2001, 68(1), 31-40.
M.N. Timofeeva; S.T. Khankhasaeva; Y.A. Chesalov; S.V. Tsybulya; V.N. Panchenko; E.T. Dashinamzhilova; Appl. Catal., B, 2009, 88(1), 127-134.
A. Gil; M. Montes; Langmuir, 1994, 10(1), 291-297.
A.-M. Georgescu; F. Nardou; I.D. Nistor; Sci. Study Res. Chem. Chem. Eng., Biotechnol., Food Ind., 2016, 17(3), 261-269.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 Studia Universitatis Babeș-Bolyai Chemia

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.