MPROVING THE CORROSION RESISTANCE OF MILD STEEL BY ZINC-GRAPHENE OXIDE COATINGS
DOI:
https://doi.org/10.24193/subbchem.2020.3.02Keywords:
Graphene Oxide, Electrochemical Impedance Spectroscopy, Polarization Curves, Corrosion Resistance, Steel CorrosionAbstract
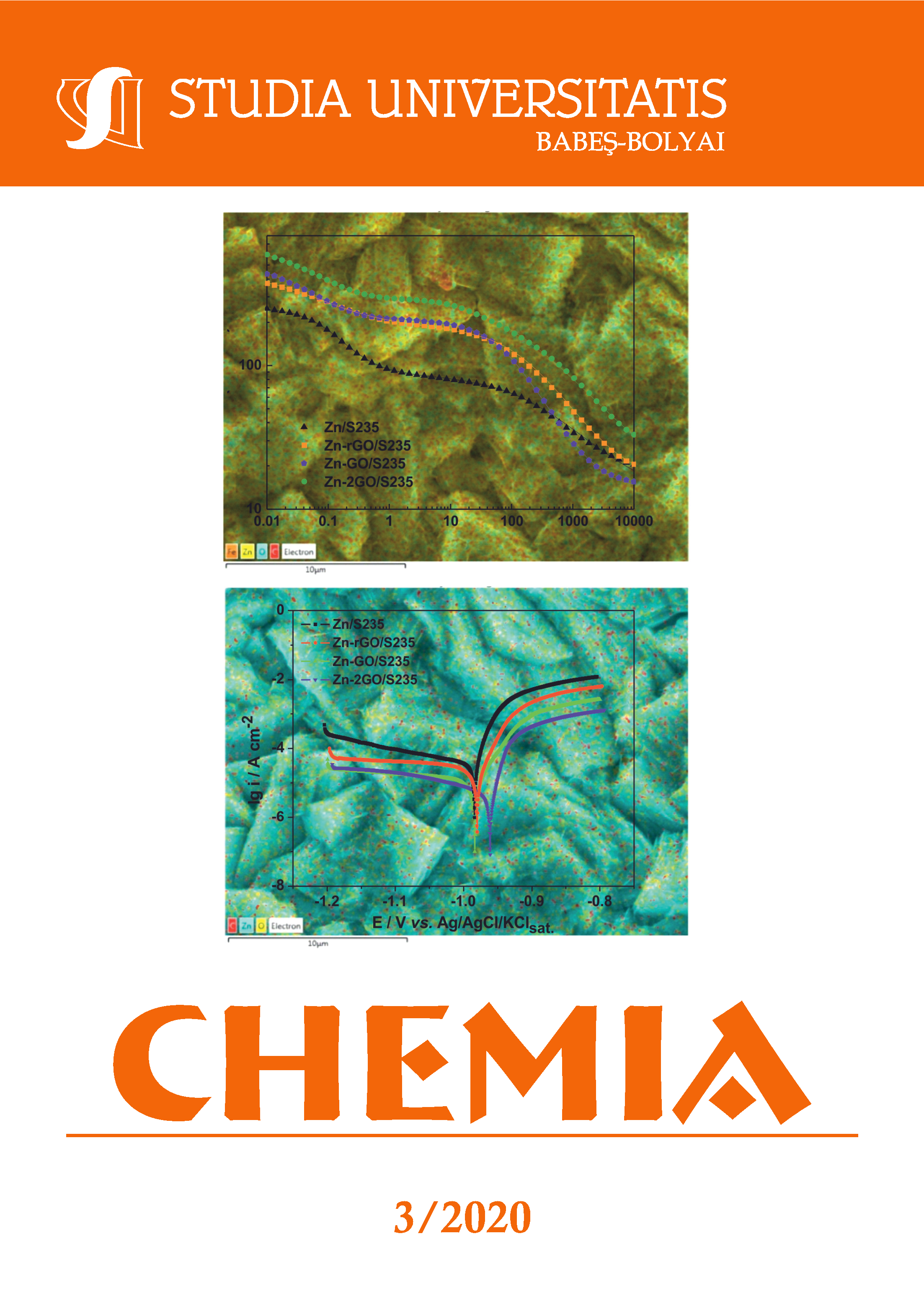
The main purpose of this work is to improve the corrosion resistance of steel substrates using the characterization of new composite zinc electrodeposits containing graphene oxides and reduced graphene oxide. The zinc-graphene based composite deposits were obtained by electrodeposition of an acidic electrolyte (pH = 5) at a current density of 20 mA/cm2. Anionic surfactant (i.e., sodium dodecyl sulfate) was used to obtain uniform and compact coating morphology. Also, the investigated deposits had in their structure the graphene oxides produced by graphite exfoliation and the results concerning corrosion behavior of the zinc electrodeposits (Zn/S235 and Zn-graphene/S235) were compared using the same experimental conditions. Microstructural characterization was carried out by SEM‐EDS, whereas corrosion resistance was evaluated by EIS and polarization curves.
References
R. Jain; R. Pitchumani; Surf. Coat. Tech., 2018, 337, 223-231.
C.T.J. Low; R.G.A. Wills; F.C. Walsh; Surf. Coat. Tech. 2006, 201, 371-383.
A. Ambrosi; M. Pumera; Chem-Eur. J., 2016, 22, 153-159.
S. K. Choudhary; A. K. Gupta; Solid State Commun., 2011, 151, 396-399.
M. Schriver; W. Regan; W.J. Gannett; A.M. Zaniewski; M.F. Crommie; A. Zettl; ACS Nano, 2013, 7, 5763-5768.
M. Yi; Z. Shen; J. Mater Chem. A., 2015, 3, 11700-11715.
G. Allaedinia; E. Mahmoudia; P. Aminayib; S. Masrinda Tasirina; A. Wahab Mohammad; Synth. Met., 2016, 220, 72-77.
X. Chen; D. Meng; B. Wang; B.W. Li; W. Li; C.W. Bielawski; R.S. Ruoff; Carbon, 2016, 101, 71-76.
A.B. López-Oyama; M.A. Domínguez-Crespo; A.M. Torres-Huerta; E. Onofre-Bustamante; R. Gámez-Corrales; N. Cayetano-Castro; Diam. Relat. Mater., 2018, 88, 167-188.
N.T. Kirkland; T. Schiller; N. Medhekar; N. Birbilis; Corros. Sci., 2012, 56, 1-4.
B.P. Singh; S. Nayak; K.K. Nanda; B.K. Jena; S. Bhattacharjee; L. Besra; Carbon, 2013, 61, 47-56.
B. Ramezanzadeh; A. Ahmadi; M. Mahdavian; Corros. Sci., 2016, 109, 182-205.
J. Zhao; X. Xie; C. Zhang; Corros. Sci., 2017, 114, 146-155.
X. Shen; J. Sheng; Q. Zhang; Q. Xu; D. Cheng, J. Mater. Eng. Perform., 2018, 27, 3750-3761.
S. Ryu; Y.J. Kwon; Y. Kim; J. Uk Lee; Mater. Chem. Phys., 2020, 250, 123039-123047.
J.D. Chelaru; D. Aylakov, L.M. Mureşan, Studia UBB Chem., 2017, 4,357-368.
A. Pérez del Pino; E. György; C. Cotet; L. Baia; C. Logofatu; RSC Advances, 2016, 6, 50034-50042.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2020 Studia Universitatis Babeș-Bolyai Chemia

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.