Thermal and Spectroscopic Investigations of Complexes of the Selected Transition Metal Ions with a β-L-Aspartyl Amide Derivative
DOI:
https://doi.org/10.24193/subbchem.2024.2.02Keywords:
L-aspartic acid, aspartyl amide, metal complexes, thermal behavior, spectroscopic studiesAbstract
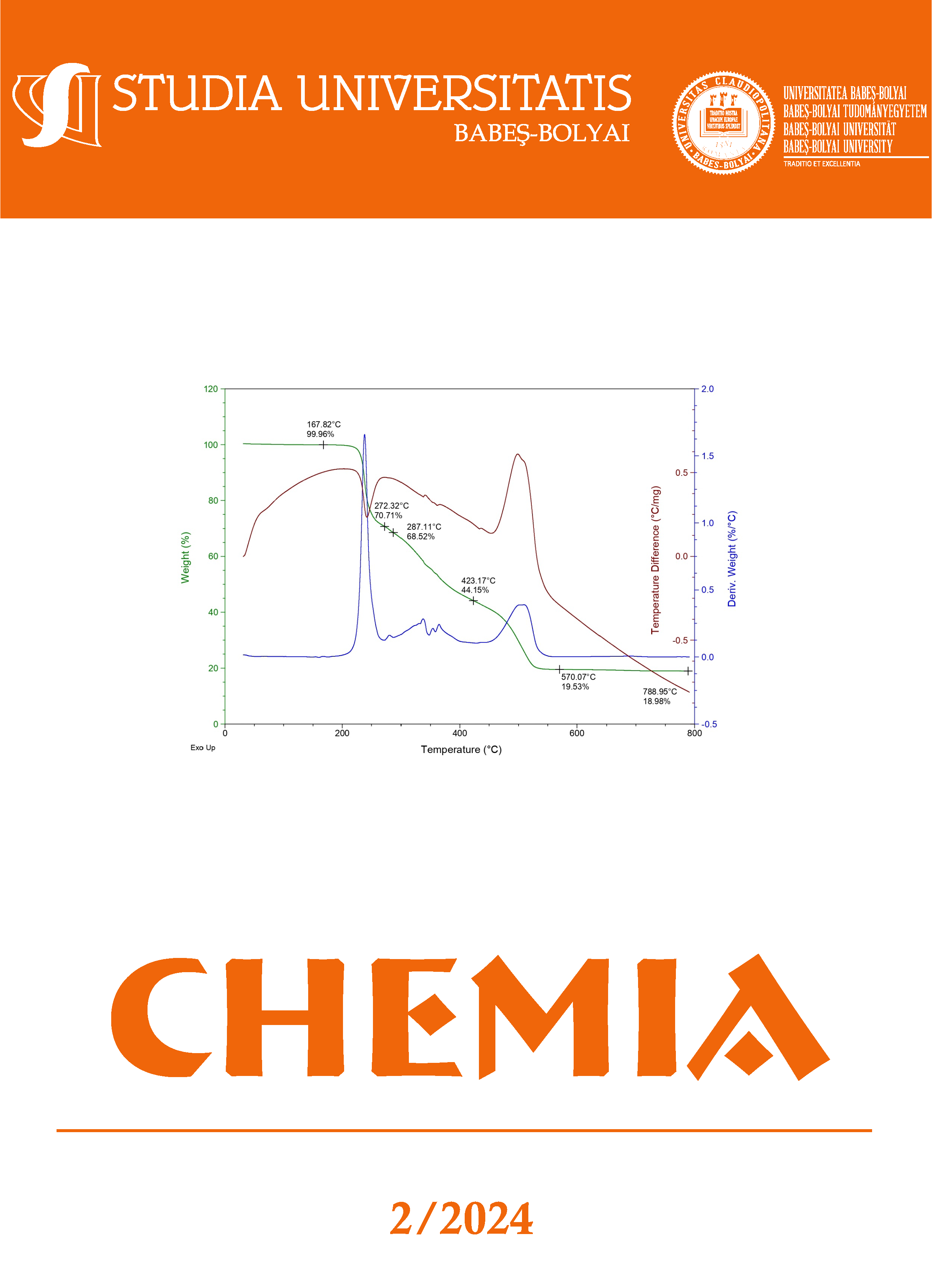
The aim of the presented research was to synthesize and characterize via elemental analysis, HRMS, thermogravimetric analysis, FTIR and EPR a novel series of transition metal complexes of Cu(II), Co(II), Ni(II) and Mn(II) with β-L-aspartyl-cyclohexyl amide as ligand. The HRMS recorded spectra confirm the obtaining of new compounds. The changes in the FTIR spectra of the metal complexes, compared to the ligand, support the complexation process. The thermal stability of the ligand and its metal complexes was discussed in the 20-8000C temperature range, in air atmosphere. In all of the studied complexes, the aspartyl amide acts as a bidentate ligand, its coordination involving the carboxylate oxygen and the nitrogen atom belonging to the free amino group of the amino acid. Metal complexes are formed in the 1:2 (Metal:Ligand) ratio as found by the elemental analysis. Except the free ligand, all the metal complexes are hydrating with water molecules, and the thermal stability of these suggested whether the water molecules are inside or outside the coordination sphere. The shape of EPR spectrum for copper complex at room temperature suggests the presence of CuN2O2 monomeric species with a rhombic distortion around the metallic ion. The results indicate that their stability range obeys the Irving-Williams series.
References
Y. M. Jamil; M. A. Al-Maqtari; F. M. Al-Azab; M. K. Al-Qadasy; A. A. Al-Gaadbi; Eclet. Quim., 2018, 43(4), 11-24
M. Khuddus; M. Jayakannan; Biomacromolecules, 2023, 24, 2643-2660
Miller; A. Matera-Witkiewicz; A. Mikolajczyk; J. Watly; D. Wilcox, D. Witkowska; M. Rowinska-Zyrek; Int. J. Mol. Sci., 2021, 22, 6971
Tomashevskii; O. A. Golovanova; S. V. Anisina; Russ. J. Gen. Chem., 2021, 91(12), 2621-2626
N. Lihi; M. Lukacs; D. Szucs; K. Varnagy; I. Sovago; Polyhedron., 2017, 133, 364-373
M. Raics; D. Sanna; I. Sovago; C. Kallay; Inorganica Chim. Acta., 2015, 426, 99-106
T. O. Aiyelabola; D. A. Isabirye; E. O. Akinkunmi; O. A. Ogunkunle; I. A. O. Ojo; J. Chem., 2016, Article ID 7317015, https://doi.org/10.1155/2016/7317015
Nomiya; H. Yokoyama; J. Chem.Soc., Dalton Trans., 2002, 12, 2483-2490
D. Kalebic; K. Binnemans; P. A. M. de Witte; W. Dehaen; RSC Sustainability, 2023, 1, 1995-2005
Y. Hui; H. Qizhuang; Z. Meifeng; X. Yanming; S. Jingyi; J. Chin. Rare Earth Soc., 2007, 2, 150-156
X. Liu; M. Wu; C. Li; P. Yu; S. Feng; Y. Li; Q. Zhang; Molecules, 2022, 27, 2407
M. Patyal; K. Kaur; N. Gupta; R. Kaur; A. K. Malik; Prot. Met. Phys. Chem., 2023, 59(2), 169-178
I. Abdiji; C. Sacalis; A. Shabani; A. Jashari; J. Nat. Sci. Math., 2023, 8(15-16), 72-81
H. Irving; R. J. P. Williams; J. Chem. Soc., 1953, 3192-3210
I. Sovago; E. Farkas; C. Bertalan; A. Lebkiri; T. Kowalik-Jankowska; H. Kozlowski; J. Inorg. Biochem., 1993, 51, 715-726
L. Zapala; M. Kosinska; E. Woznicka; L. Byczynski; W. Zapala; J. Therm. Anal. Calorim., 2016, 124, 363-374
C. Sacalis; F. Goga; C. Somesan; Studia UBB Chemia, 2018, 63(4), 51-63
C. Sacalis; F. Goga; L. David; Studia UBB Chemia, 2017, 62(4), 181-192
C. Sacalis; S. Jahiji, A. Avram; Studia UBB Chemia, 2022, 67(4), 337-352
M. Castillo; E. Ramirez; Transit. Met. Chem., 1984, 9, 268-270
Z. K. Genc; S. Selcuk; S. Sandal; N. Colak; S. Keser; M. Sekerci; M. Karatepe; Med. Chem. Res., 2014, 23, 2476-2485
P. S. Piispanen; P. M. Pihko; Tetrahedron Lett., 2005, 46, 2751-2755
A. M. Hassan; B. H. Heakal; A. O. Said; W. M. Aboulthana; M. A. Abdelmoaz; Egypt. J. Chem., 2020, 63(7), 2533-2550
W. Ferenc; B. Cristovao; J. Sarzynski; P. Sadowski; J. Therm. Anal. Calorim., 2012, 110, 739-748
I. Sakiyan; Transit. Met. Chem., 2007, 32, 131-135
M. Tanaka; I. Yamashina; Carbohydr. Res., 1973, 27, 175-183
K. Nakamoto; Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part. B: Applications in Coordination, Organometallic and Bioinorganic Chemistry, John Wiley & Sons, Inc., Hoboken, New Jersey, Sixth Ed., 2009, pp. 63-72
A. A. Osunlaja; N. P. Ndahil; J. A. Ameh; Afr. J. Biotechnol., 2009, 8(1), 4-11
O. F. Akinyele; A. B. Adesina; T. A. Ajayeoba; E. G. Fakola; Sci. J. Chem., 2023, 11(4), 137-145
T. H. Al-Noor; M. R. Aziz; A. T. AL-Jeboori; J. Chem. Pharm. Res., 2014, 6(4), 1225-1231
C. A. Terraza; L. H. Tagle; D. Munoz; A. Tundidor-Camba; P. A. Ortiz; D. Coll; C. M. Gonzalez-Henriquez; I. A. Jessop; Polym. Bull., 2016, 73, 1103-1117
M. Worzakowska; M. Sztanke; K. Sztanke; J. Therm. Anal. Calorim., 2022, 147, 14315-14327
L. D. Pinto; P. A. L. Puppin; V. M. Behring; D. H. Flinker; A. L. R. Merce; A. S. Mangrich; N. A. Rey; J. Felcman; Inorganica Chim. Acta, 2010, 363, 2624-2630
J. R. Pilbrow, Transition ion electron paramagnetic resonance, Clarendon Press, New York: Oxford University Press, 1990, pp. 51-80
F. E. Mabbs; D. Collison; Electron Paramagnetic Resonance of d Transition Metal Compounds, Elsevier Science Publishers B.V. Amsterdam, Netherland, 2013, pp. 83-124.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Studia Universitatis Babeș-Bolyai Chemia

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.