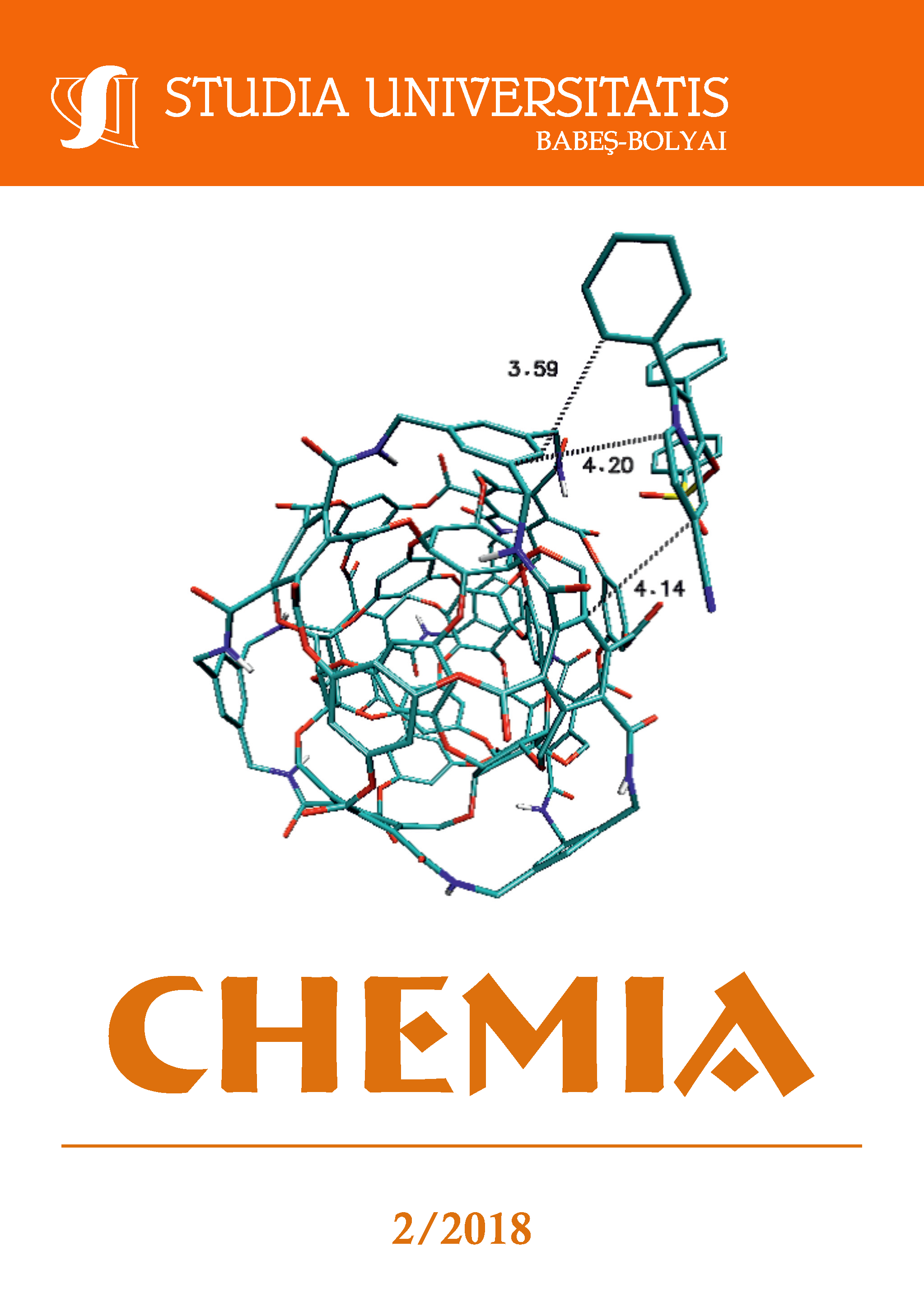
DOCKING OF INDOLIZINE DERIVATIVES ON CUBE RHOMBELLANE FUNCTIONALIZED HOMEOMORPHS
DOI:
https://doi.org/10.24193/subbchem.2018.2.01Keywords:
binding energy, indolizine, molecular docking, nanostructure, cube rhombellane homeomorphAbstract
Indolizines represent a class of heteroaromatic compounds (of pharmacological importance) containing two condensed (5- and 6-memebered) rings bridged by a nitrogen atom, showing a variety of biological activities. An attempt was made to deposit indolizines on the cube rhombellane homeomorphs surface as possible nano-drug complexes, since rhombellane homeomorphs may be bound in a protein as the active pocket and further may be used in personalized medicine. In the present study, a molecular docking analysis of two indolizine derivatives on some cube rhombellane homeomorphs was carried out for the first time.
References
Q. Long, Y. Xiel, Y. Huang et al., Journal Biomedical Nanotechnology, 2013, 9, 965.
M. Benezra, O. Penate-Medina, P.B. Zanzonico, et al. Journal of Clinical Investigation, 2011, 121, 2768.
H. Ali-Boucetta, K. T. Al-Jamal, D. McCarthy et al,. Chemical Communications, 2008, 28, 459.
S. Sánchez-Paradinas, M. Pérez-Andrés, M.J. Almendral-Parra, et al., Journal of Inorganic Biochemistry, 2014, 131, 8.
M. P. Evstigneev, A. S. Buchelnikov, D. P. Voronin, Y. V. Rubin, L. F. Belous, Y. I. Prylutskyy, U. Ritter, Chem. Phys. Chem., 2013, 14, 568.
G. S. Singh, E. E. Mmatli, European Journal of Medicinal Chemistry, 2011, 46, 5237.
S. S. Juang, M. Chang, L. F. Wang, J. L. Han, C. H. Ong, Tetrahedron, 2005, 61, 1693.
T. Przewloka, S. Chen, Z. Xia, H. Li, S. Zhang, D. Chimmanamada, E. Kostik, D. James, K. Koya, L. Sun, Tetrahedron Lett., 2007, 48, 5739.
I. V. Seregin, A. W. Schammel, V. Gevorgyan, Tetrahedron, 2008, 64, 6876.
B. Szefler, T. E. Harsa, A. M. Harsa, Studia UBB Chemia, 2015, 60, 201.
B. Szefler, P. Czeleń, M. V. Diudea, Current Computer-Aided Drug Design, 2017, 13, 22.
B. Szefler, P. Czeleń, Journal. Molecular Modeling, 2017, 23, 208.
PubChem database, accessed 10. 10. 2014.
M. V. Diudea, Intl. Conf. “Bio-Nano-Math-Chem”, 2017, Cluj, Romania.
K. B. Wiberg, F. H. Walker, [1.1.1]Propellane, Journal of American Chemical Society, 1982, 104, 5239.
P. Kazynsky, J. Michl, Journal of American Chemical Society, 1988, 110, 5225.
M. V. Diudea, Iranian Journal of Mathematical Chemistry, 2018, 9, 1.
D. R. Do Carmo, L. L. Paim, N. L. Dias Filho, N. R. Stradiotto, Applied Surface Science, 2007, 253, 3683.
D. R. Do Carmo, U. O. Bicalho, T. F. Silveira, N. L. Dias Filho, L. L. Paim, Journal of Chemistry, 2013, 2, 1.
M. V. Diudea, International Journal of Chemical Modeling, 2018, 9, 2, 000.
O. Trott, A. J. Olson, Journal Computer Chemistry, 2010, 31, 455.
B. K Shoichet, I. D. Kuntz, D. L. Bodian, Journal Computer Chemistry, 2004, 13, 380.
K. Dhananjayan, K. Kalathil, A. Sumathy, P. Sivanandy, Der Pharma Chemica, 2014, 6, 378.
R. Abagyan, M. Totrov, Current Opinion in Chemical Biology, 2001, 5, 375.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2018 Studia Universitatis Babeș-Bolyai Chemia

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.