KINETIC AND MECHANISTIC FEATURES OF THE THIOLACTIC ACID OXIDATION BY CHROMIUM(VI) IN ACIDIC ENVIRONMENT
DOI:
https://doi.org/10.24193/subbchem.2018.4.03Keywords:
kinetics, redox, chromium VI, thiolactic acidAbstract
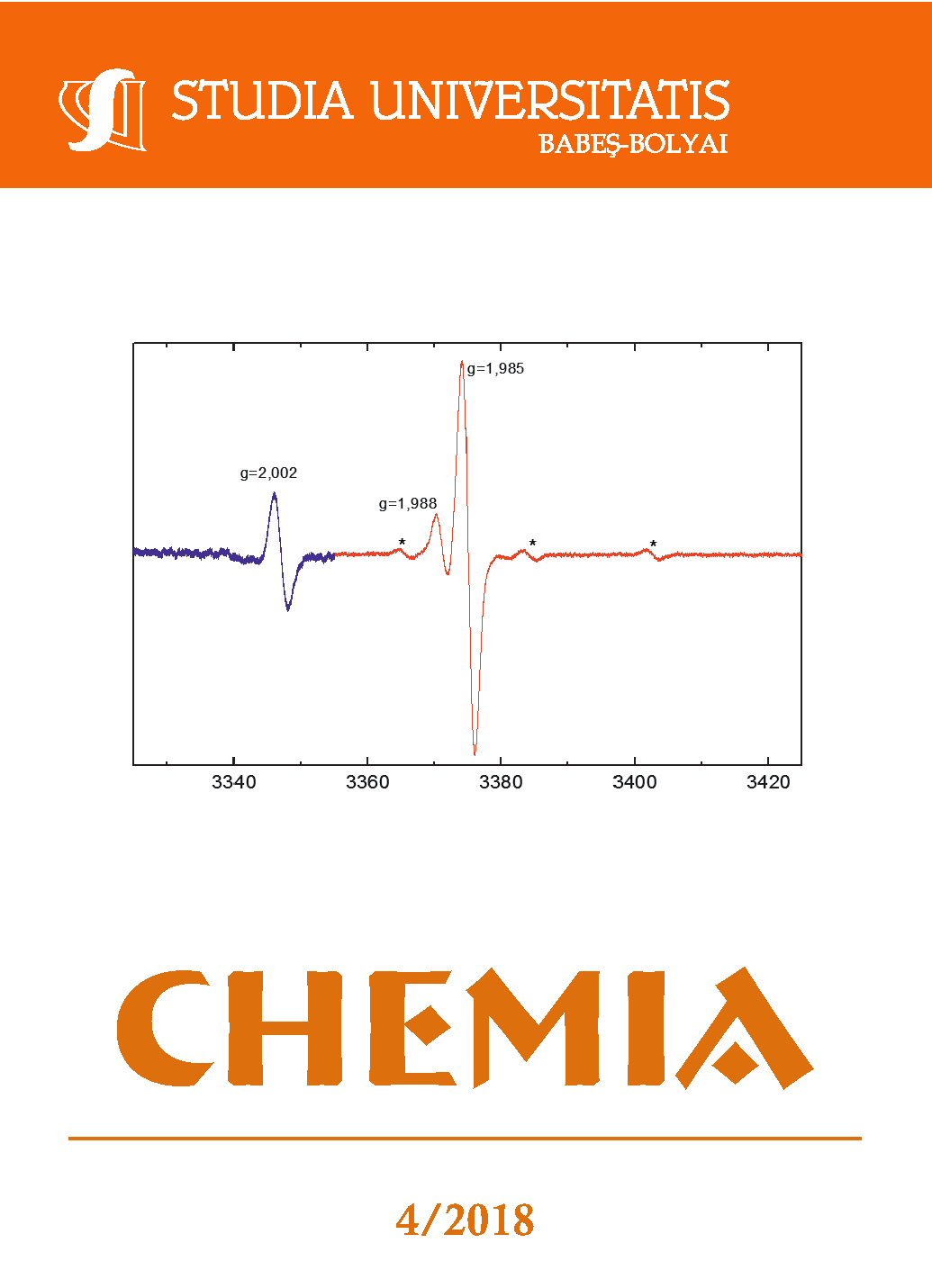
Thiolactic acid, containing a reactive sulphydryl group, has been shown to easily reduce Cr(VI) and yield disulfide as oxidation product. The reaction was studied by the use of spectrophotometry. The spectral data as well as the kinetic information showed distinct evidence of the formation of an S-bonded Cr(VI)-thiolactate intermediate that was subsequently followed by a somewhat slower, bimolecular redox process leading to the formation of the final products. The rate laws for these two stages have been determined, showing a complex dependence on substrate and hydrogen ion concentration. Small experimental Arrhenius activation energies for the two successive steps were also obtained. The involvement of paramagnetic Cr(V) species and that of some organic free radicals were evidenced by ESR and, the latter, also by initialization of polymerization. A reaction mechanism has been proposed, that leads to a rate law in convincing agreement with the experimental one.
References
V. Bianchi, A. G. Lewis, Toxicological & Environmental Chemistry, 1987, 15, 1.
P. H. Connett, K. E. Wetterhahn, "Metabolism of the carcinogen chromate by cellular constituents", In: Inorganic Elements in Biochemistry. Structure and Bonding, Springer-Verlag, Berlin, Heidelberg, 1983, 54, 93.
M. D. Cohen, B. Kargacin, C. B. Klein, M. Costa, Critical Reviews in Toxicology, 1993, 23, 255.
S. Mishra, R. N. Bharagava, Journal of Environmental Science and Health, Part C, 2016, 34, 1.
R. Saha, R. Nandi, B. Saha, Journal of Coordination Chemistry, 2011, 64, 1782.
I. Bâldea, D.-M. Sabou, Studia Universitatis Babeş-Bolyai, Chemia, 2001, 46(1-2), 17 and references therein.
U. Kläning, M. C. R. Symons, Journal of the Chemical Society, 1961, 3204.
N. Bailey, A. Carrington, K. A. K. Lott, M. C. R. Symons, Journal of the Chemical Society, 1960, 290.
M. Cohen, F. H. Westheimer, Journal of the American Chemical Society, 1952, 74, 4387.
A. McAuley, M. A. Olatunji, Canadian Journal of Chemistry, 1977, 55, 3328; ibid., 1977, 55, 3335.
G. P. Haight, E. Perchonock, P. Emmenegger, G. Gordon, Journal of the American Chemical Society, 1965, 87, 3835.
K. B. Wiberg, H. Schäfer, Journal of the American Chemical Society, 1969, 91, 933.
J. H. Espenson, Accounts of Chemical Research, 1970, 3(10), 347.
V. P. Roldán, V. A. Daier, B. Goodman, M. I. Santoro, J. C. González, N. Calisto, S. R. Signorella, L. F. Sala, Helvetica Chimica. Acta, 2000, 83, 3211.
D. A. Dixon, N. P. Sadler, T. P. Dasgupta, Journal of the Chemical Society, Dalton Transactions, 1993, 23, 3489.
G. P. Haight, G. M. Jursich, M. T. Kelso, P. J. Merill, Inorganic Chemistry, 1985, 24, 2740.
J. F. Perez-Benito, C Arias, R. M. Rodriguez, Journal of Physical Chemistry A, 2001, 105, 1150.
S. Signorella, M. I. Frascaroli, S. García, M. Santoro, J. C. González, C. Palopoli, V. Daier, N. Casado, L. F. Sala, Journal of the Chemical Society, Dalton Transactions, 2000, 1617.
S. N. Mahapatro, M. Krumpolc, J. Roček, Journal of the American Chemical Society, 1980, 102, 3799.
P. Subramaniam, N. T. Selvi, American Journal of Analytical Chemistry, 2013, 4, 20.
D. C. Ramdon, D. A. Dixon, T. P. Dasgupta, Inorganic Reaction Mechanisms, 2004, 5(3), 1.
D.-M. Sabou, Studia Universitatis Babeş-Bolyai, Chemia,, 2014, 59(4), 183.
J. Y. Tong, E. L. King, Journal of the American Chemical Society, 1953, 75, 6180.
N. N. Greenwood, A. Earnshaw, ”Chemistry of the Elements”, 2. Edition, Butterworth-Heinemann, Oxford, UK, 1997, chapter 23.
D.-M. Sabou, A. Csavdári, Muszaki Szemle (Tehnical Review), 2015, 66, 26.
T. Carrington, International Journal of Chemical Kinetics, 1982, 14(5), 517.
I. Bâldea, D.-M. Sabou, A. Csavdari, Studia Universitatis Babeş-Bolyai, Chemia, 2007, 52(1), 19.
A. Csavdári, I. Bâldea, D.-M. Sabou, Studia Universitatis Babeş-Bolyai, Chemia, 2007, 52(3), 113.
A. Levina, L. Zhang, P. A. Lay, Journal of the American Chemical Society, 2010, 132, 8720 and references therein.
Z. M. Hoffman, E. Hayon, Journal of the American Chemical Society, 1972, 94(23), 7950.
I. Bâldea, D.-M. Sabou, A Csavdári, Revue Roumaine de Chimie, 2009, 54(10), 791.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2018 Studia Universitatis Babeș-Bolyai Chemia

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.